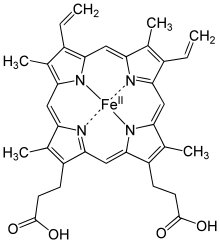
Structure of heme
Hemoglobin has a quaternary structure characteristic of many multi-subunit globular proteins. Most of the amino acids in hemoglobin form alpha helices, and these helices are connected by short non-helical segments. Hydrogen bonds stabilize the helical sections inside this protein, causing attractions within the molecule, which then causes each polypeptide chain to fold into a specific shape. Hemoglobin's quaternary structure comes from its four subunits in roughly a tetrahedral arrangement.
In most vertebrates, the hemoglobin molecule is an assembly of four globular protein subunits. Each subunit is composed of a protein chain tightly associated with a non-protein prosthetic heme group. Each protein chain arranges into a set of alpha-helix structural segments connected together in a globin fold arrangement. Such a name is given because this arrangement is the same folding motif used in other heme/globin proteins such as myoglobin. This folding pattern contains a pocket that strongly binds the heme group.
A heme group consists of an iron (Fe) ion held in a heterocyclic ring, known as a porphyrin. This porphyrin ring consists of four pyrrole molecules cyclically linked together (by methine bridges) with the iron ion bound in the center. The iron ion, which is the site of oxygen binding, coordinates with the four nitrogen atoms in the center of the ring, which all lie in one plane. The iron is bound strongly (covalently) to the globular protein via the N atoms of the imidazole ring of F8 histidine residue (also known as the proximal histidine) below the porphyrin ring. A sixth position can reversibly bind oxygen by a coordinate covalent bond, completing the octahedral group of six ligands. This reversible bonding with oxygen is why hemoglobin is so useful for transporting oxygen around the body. Oxygen binds in an "end-on bent" geometry where one oxygen atom binds to Fe and the other protrudes at an angle. When oxygen is not bound, a very weakly bonded water molecule fills the site, forming a distorted octahedron.
Even though carbon dioxide is carried by hemoglobin, it does not compete with oxygen for the iron-binding positions but is bound to the amine groups of the protein chains attached to the heme groups.
The iron ion may be either in the ferrous Fe2+ or in the ferric Fe3+ state, but ferrihemoglobin (methemoglobin) (Fe3+) cannot bind oxygen. In binding, oxygen temporarily and reversibly oxidizes (Fe2+) to (Fe3+) while oxygen temporarily turns into the superoxide ion, thus iron must exist in the +2 oxidation state to bind oxygen. If superoxide ion associated to Fe3+ is protonated, the hemoglobin iron will remain oxidized and incapable of binding oxygen. In such cases, the enzyme methemoglobin reductase will be able to eventually reactivate methemoglobin by reducing the iron center.
In adult humans, the most common hemoglobin type is a tetramer (which contains four subunit proteins) called hemoglobin A, consisting of two α and two β subunits non-covalently bound, each made of 141 and 146 amino acid residues, respectively. This is denoted as α2β2. The subunits are structurally similar and about the same size. Each subunit has a molecular weight of about 16,000 daltons, for a total molecular weight of the tetramer of about 64,000 daltons (64,458 g/mol). Thus, 1 g/dL = 0.1551 mmol/L. Hemoglobin A is the most intensively studied of the hemoglobin molecules.
In human infants, the hemoglobin molecule is made up of 2 α chains and 2 γ chains. The gamma chains are gradually replaced by β chains as the infant grows.
The four polypeptide chains are bound to each other by salt bridges, hydrogen bonds, and the hydrophobic effect.
Oxygen saturationedit
In general, hemoglobin can be saturated with oxygen molecules (oxyhemoglobin), or desaturated with oxygen molecules (deoxyhemoglobin).
Oxyhemoglobinedit
Oxyhemoglobin is formed during physiological respiration when oxygen binds to the heme component of the protein hemoglobin in red blood cells. This process occurs in the pulmonary capillaries adjacent to the alveoli of the lungs. The oxygen then travels through the blood stream to be dropped off at cells where it is utilized as a terminal electron acceptor in the production of ATP by the process of oxidative phosphorylation. It does not, however, help to counteract a decrease in blood pH. Ventilation, or breathing, may reverse this condition by removal of carbon dioxide, thus causing a shift up in pH.
Hemoglobin exists in two forms, a taut (tense) form (T) and a relaxed form (R). Various factors such as low pH, high CO2 and high 2,3 BPG at the level of the tissues favor the taut form, which has low oxygen affinity and releases oxygen in the tissues. Conversely, a high pH, low CO2, or low 2,3 BPG favors the relaxed form, which can better bind oxygen. The partial pressure of the system also affects O2 affinity where, at high partial pressures of oxygen (such as those present in the alveoli), the relaxed (high affinity, R) state is favoured. Inversely, at low partial pressures (such as those present in respiring tissues), the (low affinity, T) tense state is favoured. Additionally, the binding of oxygen to the iron(II) heme pulls the iron into the plane of the porphyrin ring, causing a slight conformational shift. The shift encourages oxygen to bind to the three remaining heme units within hemoglobin (thus, oxygen binding is cooperative).
Deoxygenated hemoglobinedit
Deoxygenated hemoglobin is the form of hemoglobin without the bound oxygen. The absorption spectra of oxyhemoglobin and deoxyhemoglobin differ. The oxyhemoglobin has significantly lower absorption of the 660 nm wavelength than deoxyhemoglobin, while at 940 nm its absorption is slightly higher. This difference is used for the measurement of the amount of oxygen in a patient's blood by an instrument called a pulse oximeter. This difference also accounts for the presentation of cyanosis, the blue to purplish color that tissues develop during hypoxia.
Deoxygenated hemoglobin is paramagnetic; it is weakly attracted to magnetic fields. In contrast, oxygenated hemoglobin exhibits diamagnetism, a weak repulsion from a magnetic field.
Comments
Post a Comment